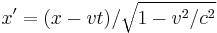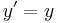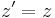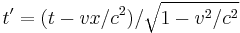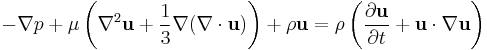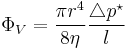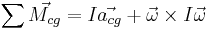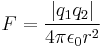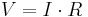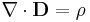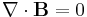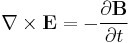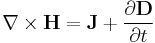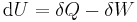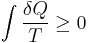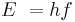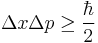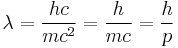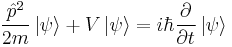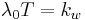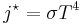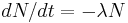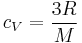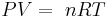Laws of science
The laws of science are various established scientific laws, or physical laws as they are sometimes called, that are considered universal and invariable facts of the physical universe. Laws of science may, however, be disproved if new facts or evidence contradicts them. A "law" differs from hypotheses, theories, postulates, principles, etc., in that a law is an analytic statement, usually with an empirically determined constant. A theory may contain a set of laws, or a theory may be implied from an empirically determined law.
Contents |
Incomplete list of scientific laws
|
Laws of motion
Laws of electromagnetism and gravitation |
Laws of energy Laws of heat transfer |
Gas laws
Laws of Aerodynamics |
Conservation laws
Most significant laws in science are conservation laws. These fundamental laws follow from homogeneity of space, time and phase (see Emmy Noether theorem).
- Conservation of mass law
- Conservation of energy law
- Conservation of momentum law
- Conservation of angular momentum law
- Charge conservation law
Relativity
- Special relativity
- Constancy of the speed of light
- Lorentz transformations – Transformations of Cartesian coordinates between relatively moving reference frames.
- Mass-energy equivalence
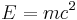 (energy = mass × speed of light2)
(energy = mass × speed of light2)
- General relativity
- Energy-momentum (including mass via E=mc2) curves spacetime.
- This is described by the Einstein field equations:
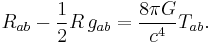
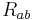 is the Ricci tensor,
is the Ricci tensor, 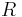 is the Ricci scalar,
is the Ricci scalar, 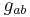 is the metric tensor,
is the metric tensor, 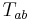 is the stress-energy tensor, and the constant is given in terms of
is the stress-energy tensor, and the constant is given in terms of 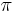 (pi),
(pi), 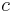 (the speed of light) and
(the speed of light) and 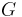 (the gravitational constant).
(the gravitational constant).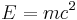 where
where 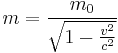
- Energy-momentum (including mass via E=mc2) curves spacetime.
Laws of classical mechanics
They are low-limit solutions to relativity. Alternative formulations of Newtonian mechanics are Lagrangian and Hamiltonian mechanics. Euler's laws of motion are extensions of Newton's laws.
-
- Law of inertia
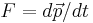 . When the mass is constant, this implies
. When the mass is constant, this implies 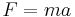 .
.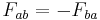 . Force of a on b equals the negative force of b on a, or for every action there is an equal but opposite reaction.
. Force of a on b equals the negative force of b on a, or for every action there is an equal but opposite reaction.
- Poiseuille's law (voluminal laminar stationary flow of incompressible uniform viscous liquid through a cylindrical tube with the constant circular cross-section)
Other
- Newton-Euler law (rotation of rigid bodies)
Classical laws of gravitation
(for modern laws see General relativity above)
- Kepler's laws of planetary motion
- Newton's law of universal gravitation – gravitational force between two objects equals the gravitational constant times the product of the masses divided by the distance between them squared. Newton's second law was gravity's effect on us humans.
-
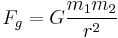
- This law is the low limit solution of Einstein's field equations and is not accurate with modern high precision gravitational measurements.
Electromagnetic laws
Pre-Maxwell laws
- Coulomb's law – Force between any two charges is equal to the product of the charges divided by 4 pi times the vacuum permittivity times the distance squared between the two charges.
Electric and magnetic fields unified:
|
Thermodynamic laws
Laws of thermodynamics
-
- Zeroth law of thermodynamics
- If two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with one another.
- First law of thermodynamics
- The change in energy dU in a system is accounted for entirely by the heat δQ absorbed by the system and the work δW done by the system:
- The change in energy dU in a system is accounted for entirely by the heat δQ absorbed by the system and the work δW done by the system:
- Second law of thermodynamics
- Third law of thermodynamics
- As the temperature T of a system approaches absolute zero, the entropy S approaches a minimum value C: as T → 0, S → C.
- Onsager reciprocal relations – sometimes called the Fourth Law of Thermodynamics
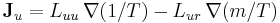 ;
;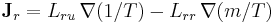 .
.
- Zeroth law of thermodynamics
Other
- Newton's law of heat conduction
- Fourier's law
Quantum laws
-
- Planck–Einstein law for the energy of photons – Energy equals Planck's constant multiplied by the frequency of the light.
- Heisenberg uncertainty principle – Uncertainty in position multiplied by uncertainty in momentum is equal to or greater than the reduced Planck constant divided by 2.
- Matter wavelength – Laid the foundations of particle-wave duality and was the key idea in the Schrödinger equation.
- Schrödinger equation – Describes the time dependence of a quantum mechanical system.
- or more compactly
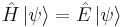
-
- The Hamiltonian (in quantum mechanics) H is a self-adjoint operator acting on the state space,
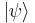 is the instantaneous quantum state vector at time t, position r, i is the unit imaginary number,
is the instantaneous quantum state vector at time t, position r, i is the unit imaginary number, 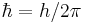 is the reduced Planck's constant. Note that
is the reduced Planck's constant. Note that 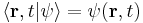 , see Dirac notation.
, see Dirac notation.
- The Hamiltonian (in quantum mechanics) H is a self-adjoint operator acting on the state space,
It is thought that the successful integration of Einstein's field equations with the uncertainty principle and Schrödinger equation, something no one has achieved so far with a testable theory, will lead to a theory of quantum gravity, the most basic physical law sought after today.
Radiation laws
Laws of electromagnetic radiation and light:
- Planck's law of black body radiation (spectral density in radiation of a black-body)
- Wien's law (wavelength of the peak of the emission of a black body)
- Stefan-Boltzmann law (total radiation from a black body)
- Beer-Lambert law (light absorption)
- Radioactive decay law (number of atoms in a radionuclide)
Laws of chemistry
Chemical laws are those laws of nature relevant to chemistry. The most fundamental concept in chemistry is the law of conservation of mass, which states that there is no detectable change in the quantity of matter during an ordinary chemical reaction. Modern physics shows that it is actually energy that is conserved, and that energy and mass are related; a concept which becomes important in nuclear chemistry. Conservation of energy leads to the important concepts of equilibrium, thermodynamics, and kinetics.
Additional laws of chemistry elaborate on the law of conservation of mass. Joseph Proust's law of definite composition says that pure chemicals are composed of elements in a definite formulation; we now know that the structural arrangement of these elements is also important.
Dalton's law of multiple proportions says that these chemicals will present themselves in proportions that are small whole numbers (i.e. 1:2 O:H in water); although in many systems (notably biomacromolecules and minerals) the ratios tend to require large numbers, and are frequently represented as a fraction.
More modern laws of chemistry define the relationship between energy and transformations.
- In equilibrium, molecules exist in mixture defined by the transformations possible on the timescale of the equilibrium, and are in a ratio defined by the intrinsic energy of the molecules—the lower the intrinsic energy, the more abundant the molecule.
- Transforming one structure to another requires the input of energy to cross an energy barrier; this can come from the intrinsic energy of the molecules themselves, or from an external source which will generally accelerate transformations. The higher the energy barrier, the slower the transformation occurs.
- There is a hypothetical intermediate, or transition structure, that corresponds to the structure at the top of the energy barrier. The Hammond–Leffler postulate states that this structure looks most similar to the product or starting material which has intrinsic energy closest to that of the energy barrier. Stabilizing this hypothetical intermediate through chemical interaction is one way to achieve catalysis.
- All chemical processes are reversible (law of microscopic reversibility) although some processes have such an energy bias, they are essentially irreversible.
- Avogadro's law (Equal volumes of ideal or perfect gases, at the same temperature and pressure, contain the same number of particles, or molecules.)
- Dulong–Petit law (specific heat capacity at constant volume)
Gas laws
Other less significant (non fundamental) laws are the mathematical consequences of the above conservation laws for derivative physical quantities (mathematically defined as force, pressure, temperature, density, force fields, etc.):
- Boyle's law (pressure and volume of ideal gas)
- Law of Charles and Gay-Lussac (gases expand equally with the same change of temperature)
- Ideal gas law
Other laws
- Buys-Ballot's law (wind travels counterclockwise around low pressure systems in the Northern Hemisphere)
See also
- List of laws
- Physical law – includes discussion of what constitutes a law
- List of scientific laws named after people
Notes
External Links
- Physics Formulary, a useful book in different formats containing many or the physical laws and formulae.
- Eformulae.com, website containing most of the formulae in different disciplines.